standard molar enthalpy of formation
The standard enthalpy of formation or standard heat of formation of a compound is referred to as the change of enthalpy during the formation of one mole of the substance from. The standard molar enthalpy of formation Delta H_frmcirc is the theoretical enthalpy change when one mole of a compound in its standard state the in the symbol at.
 |
| Various Enthalpy Change Definitions |
Table 1 Standard molar enthalpies of reaction of the ZnO nanosheets and bulk ZnO reaction systems.
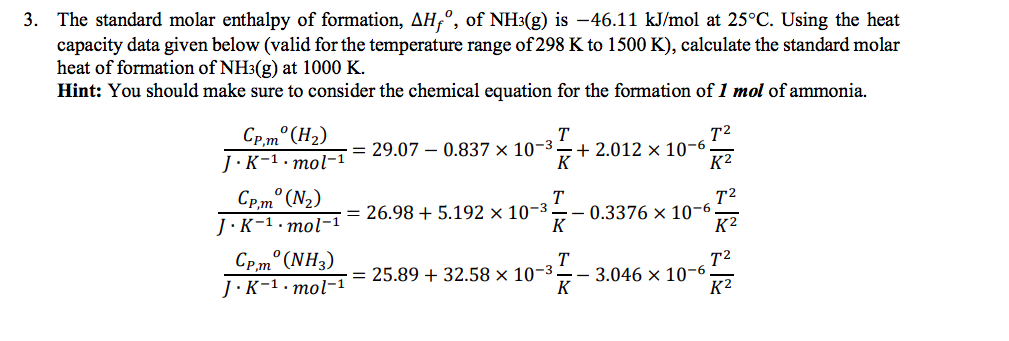
. Standard enthalpy change of formation data table These tables include heat of formation data gathered from a variety of sources including the primary and secondary literature as well as. Note that the standard. The standard enthalpy of formation of gaseous molecular iodine is 624 mathrmkJ mathrmmol. Click hereto get an answer to your question Standard molar enthalpy of formation of CO2 is equal to.
H 2 g C l 2 g H C l g By referring to the above table of thermodynamic data we can find the enthalpy of formation of the reactants under standard conditions. The standard molar enthalpy of formation is the quantity of energy that is absorbed or released when one mole of a compound is formed directly from its elements in their standard states. Solve Study Textbooks Guides. 4 rows Now we have 1 mol at -16757 kJmol as our sum of products minus 1 mol at -8242 kJmol as.
Use this information to calculate the molar heat of subli. N2g 2O2 2NO2g ΔH664kJ2NOg O2 2NO2g ΔH1141kJOpenSt. 3 rows Standard Enthalpy of Formation. EqDelta Hcirc_f eq is the enthalpy change when 1.
The standard heat of combustion of graphite. 1 La 16 V 16 O 64 Eu La 15 EuV 16 O 64 La. 393293 and 1108kJ mol1 respectively. I calculated enthalpy of formation in the following ways.
The standard enthalpy of formation of a substance is the enthalpy change that occurs when 1 mole of the substance is formed from its constituent elements in their standard states. Join Login Class 11 Chemistry. 222 Standard Enthalpy of Formation. Standard enthalpy of formation or heat of formation ΔH o f is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states.
As per the above. Standard molar enthalpy of formation of vanillin in the gaseous state was determined to be 3758 21 kJ mol 1 from ab initio calculations using density functional. The standard enthalpy of formation ΔH 0 f of a compound is the change in enthalpy that. Now I calculated the enthalpy of formation of this doped system.
Also called standard enthalpy of formation the molar heat of formation of a compound ΔH f is equal to its enthalpy change ΔH when one mole of a compound is formed. Here is the expression. The example of the formation of hydrogen bromide from bromine and hydrogen can be the best example. Given the standard enthalpy of combustion of carbon s sulphur s carbon-di-sulphide l are.
As reported in the literature 20 the standard molar. The standard molar enthalpy of formation of a compound is defined as the enthalpy of formation of 10 mol of the pure compound in its stable state. The standard molar enthalpy of formation is the quantity of energy that is absorbed or released when one mole of a compound is formed directly from its elements in their standard states. The standard p 01 MPa molar enthalpy of combustion at the temperature 29815 K of the liquid ethyl 3-oxobutanoate was determined.
H 2 g Br 2 r H o - 7281kJmol-1. Calculate the standard molar enthalpy of formation of NOg from the following data. Δ cH m -314976 076 kJmol. Standard po 01 MPa molar enthalpy of formation in both crystallineliquid and gaseous phases and standard molar enthalpy of sublimationvaporization at T 29815 K.
 |
| Enthalpies Of Formation Chemsitry Tutorial Youtube |
 |
| Standard Molar Enthalpy Of Formation Delta F H 8 Is Just A Special Case Of Enthalpy Of Reaction Delta R H 8 Is The Delta R H 8 Given Reason For Your Answer Cao S Co 2 G |
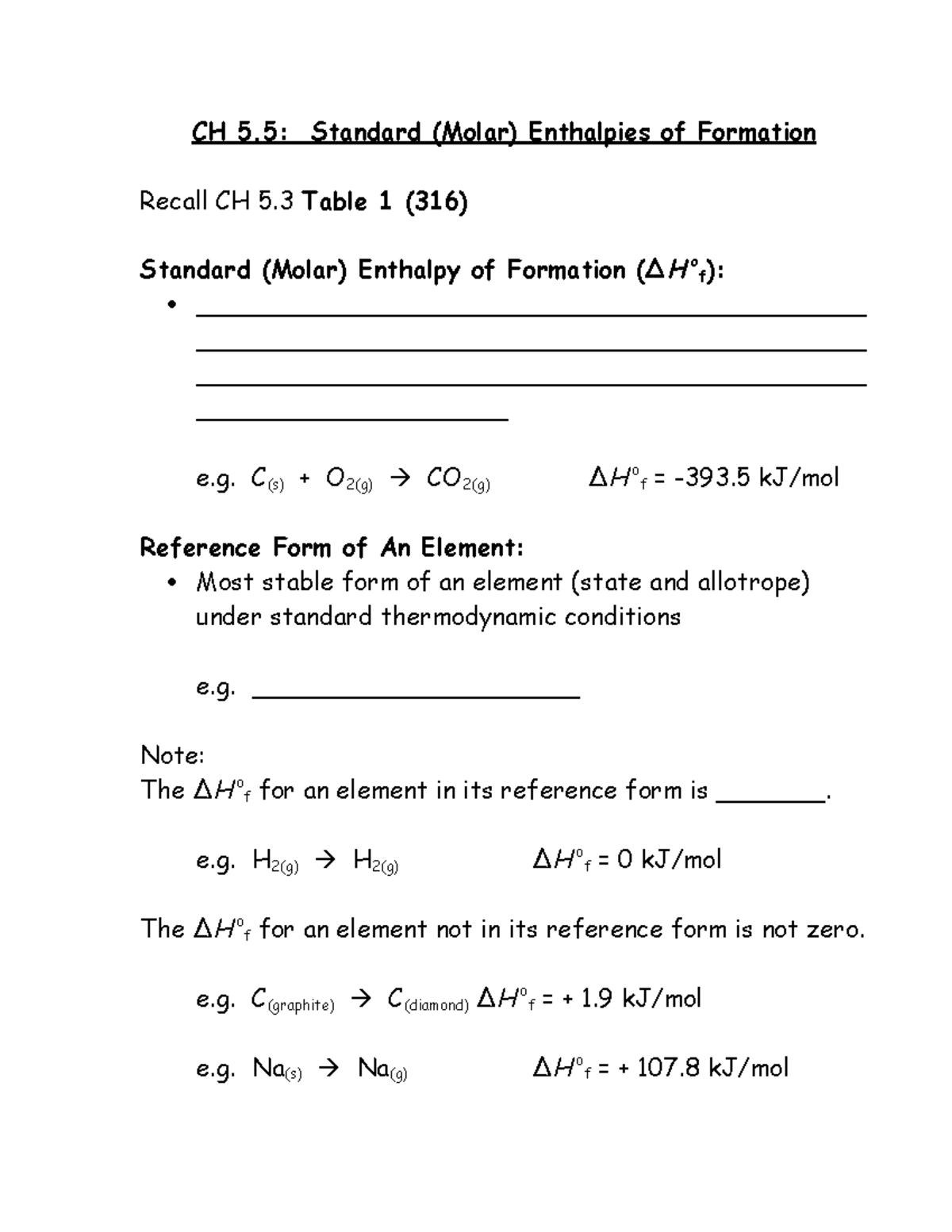 |
| Sec 5 5 Standard Enthalpies Of Formation Hand Out Ch 5 Standard Molar Enthalpies Of Formation Studocu |
 |
| Calculating The Molar Enthalpy Of Reaction From Formation Enthalpies Chemistry Study Com |
 |
| Question Video Determining The Standard Enthalpy Of Formation Of Ethanol Using Standard Enthalpies Of Combustion Nagwa |
Posting Komentar untuk "standard molar enthalpy of formation"